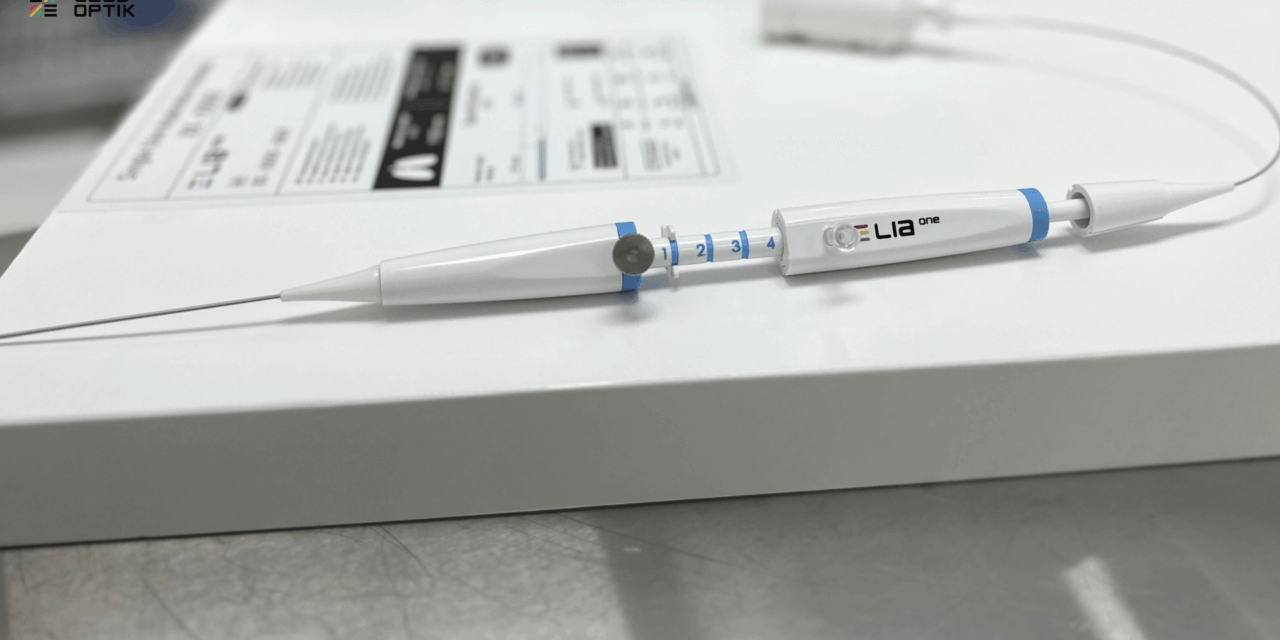
The system integrates depth imaging technology directly into biopsy tools to improve tissue sampling accuracy during bronchoscopy.
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Leadoptik Inc’s Last Inch Assessment (LIA) system, which uses silicon photonics imaging technology to enhance lung biopsy accuracy during bronchoscopy procedures.
The San Jose, California-based company says the system addresses a critical gap in lung cancer diagnosis by providing real-time optical imaging at the point of care with 50 times better resolution than current technologies. The system integrates proprietary silicon metamaterials for depth imaging directly into standard biopsy tools.
Lung cancer causes approximately 1.8 million deaths annually worldwide, making it the leading cause of cancer-related death. Early diagnosis can increase five-year survival rates by up to 94 times compared to late-stage detection, heavily depending on obtaining precise, high-quality tissue samples.
“The LIA system represents the missing link in the bronchoscopy workflow,” says George Cheng, MD, a member of Leadoptik’s clinical advisory board, in a release. “For the first time, high-resolution depth imaging is integrated directly into the biopsy tool, allowing physicians to see and characterize tissue from within.”
Addressing the “Last Inch” Challenge
While robotic and navigational bronchoscopy systems have advanced significantly in reaching pulmonary nodules, successful navigation does not guarantee diagnostic success, according to a release from the company. The final step of ensuring the tool actually samples target tissue remains challenging with current methods.
“The ‘last inch’ is where the path to timely intervention is ultimately determined,” says Dr Ali Sadoughi in a release. “Being close to a lung nodule is not enough, confirming that the needle has sampled the correct tissue is the critical missing step.”
The LIA system converts lung biopsy into what the company calls a digital, tissue-intelligence workflow. Current approaches rely on external imaging or post-procedure validation, which can add complexity and delays to the diagnostic process.
Clinical Performance Data
Preclinical data supporting the FDA clearance demonstrated biopsy accuracy exceeding 95%, according to Leadoptik. The clearance covers the consumable biopsy tool, imaging console, and associated software.
“We are in a uniquely strong position to address one of the most critical unmet needs in lung cancer diagnostics,” says Reza Khorasaninejad, CEO and co-founder of Leadoptik, in a release. “As we prepare the system for hospital deployment, our focus is on empowering physicians with real-time insight, especially in borderline cases where imaging interpretation becomes the deciding factor.”
The company’s roadmap includes developing AI-driven tissue interpretation capabilities to convert imaging data into immediate, actionable insights during interventional procedures.
Leadoptik is preparing for a limited launch of the system at select pilot sites as it advances toward broader hospital deployment.
Photo caption: Last Inch Assessment system
Photo credit: Leadoptik Inc